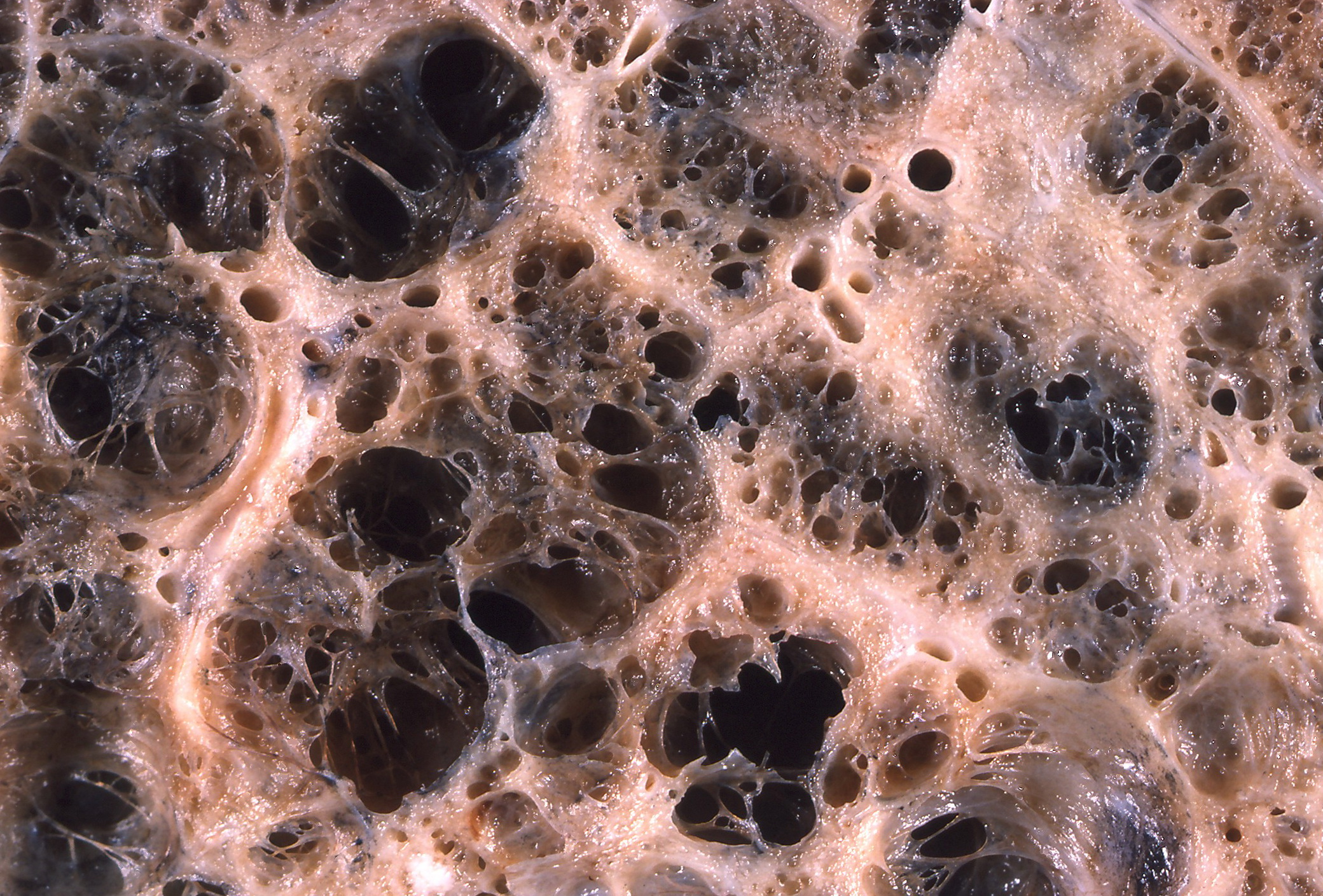
Motivation
The structural integrity and elasticity of the lungs are largely determined by the spatial arrangement of extracellular matrix protein fibers (primarily collagen and elastin) and their interconnections.
Our lab seeks to advance the understanding of fundamental lung mechanics and structure-function design even at the smallest size scales of these fiber networks — with implications for how these structures fail in diseases such as emphysema.
We investigate the dynamic mechanical properties of collagen and elastin fibers — and the networks they form — that contribute elasticity to the lungs and shape alveolar deformations.
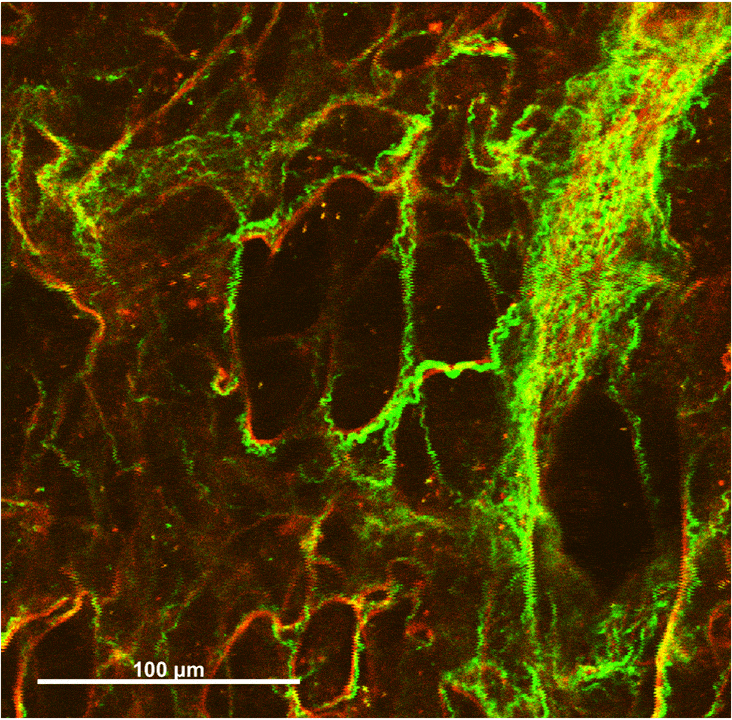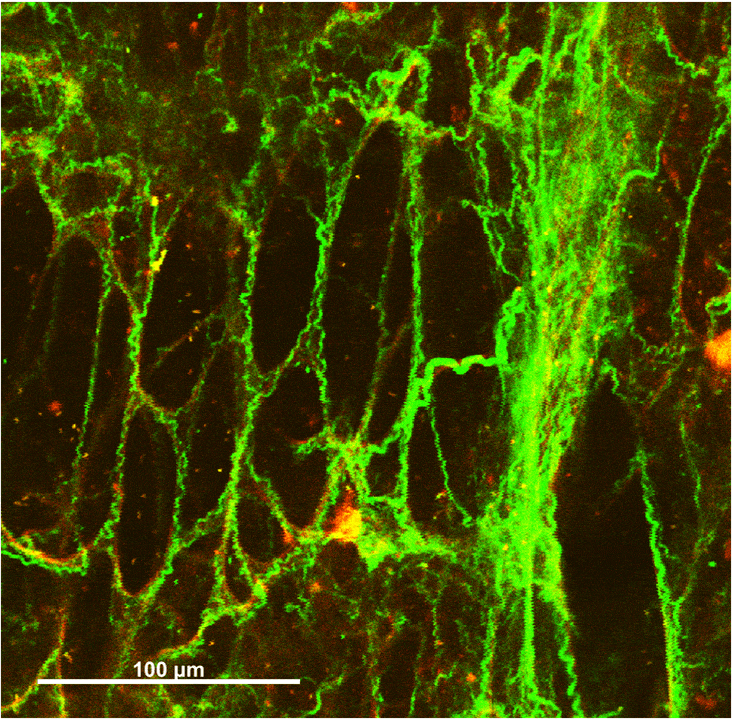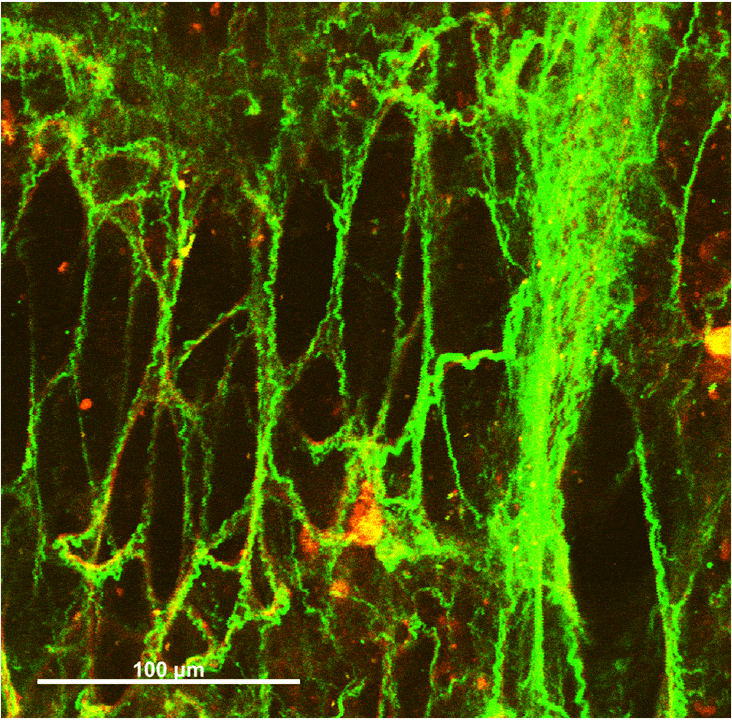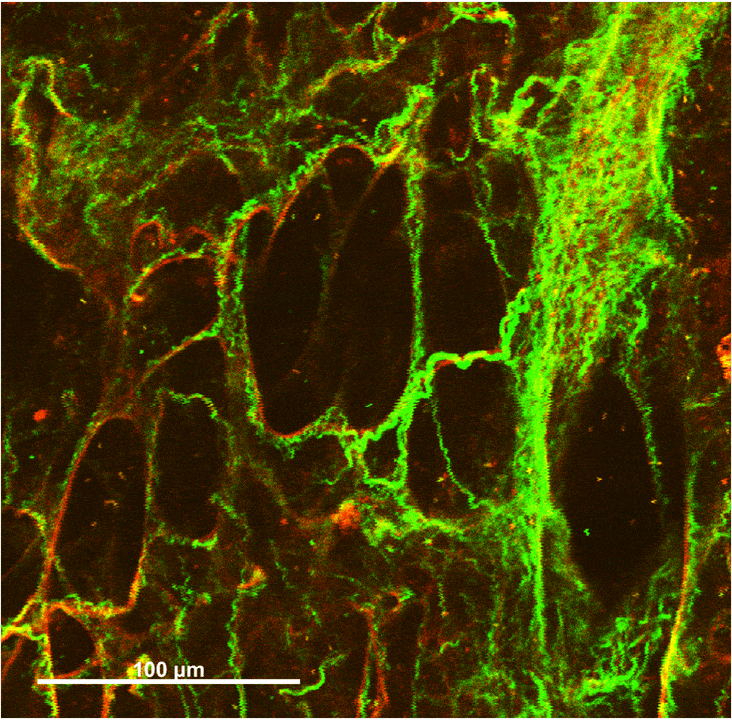
Collagen-Elastin Fiber Networks
Wavy collagen fibers (green) and taut elastin fibers (red) interact in pairs, especially around the ring-like alveolar openings. Elastin is highly distensible and confers elasticity to the lungs, while collagen is stiff and provides protection against overstretching.
Under small strains, the elastin fibers bear the majority of stress in the network, maintaining the collagen fibers in a wavy state. Fibers become aligned and compacted during uniaxial stretching. Some collagen fibers are initially wavy before stretching, but elongate until taut and recruit into a prominent stress-bearing role.
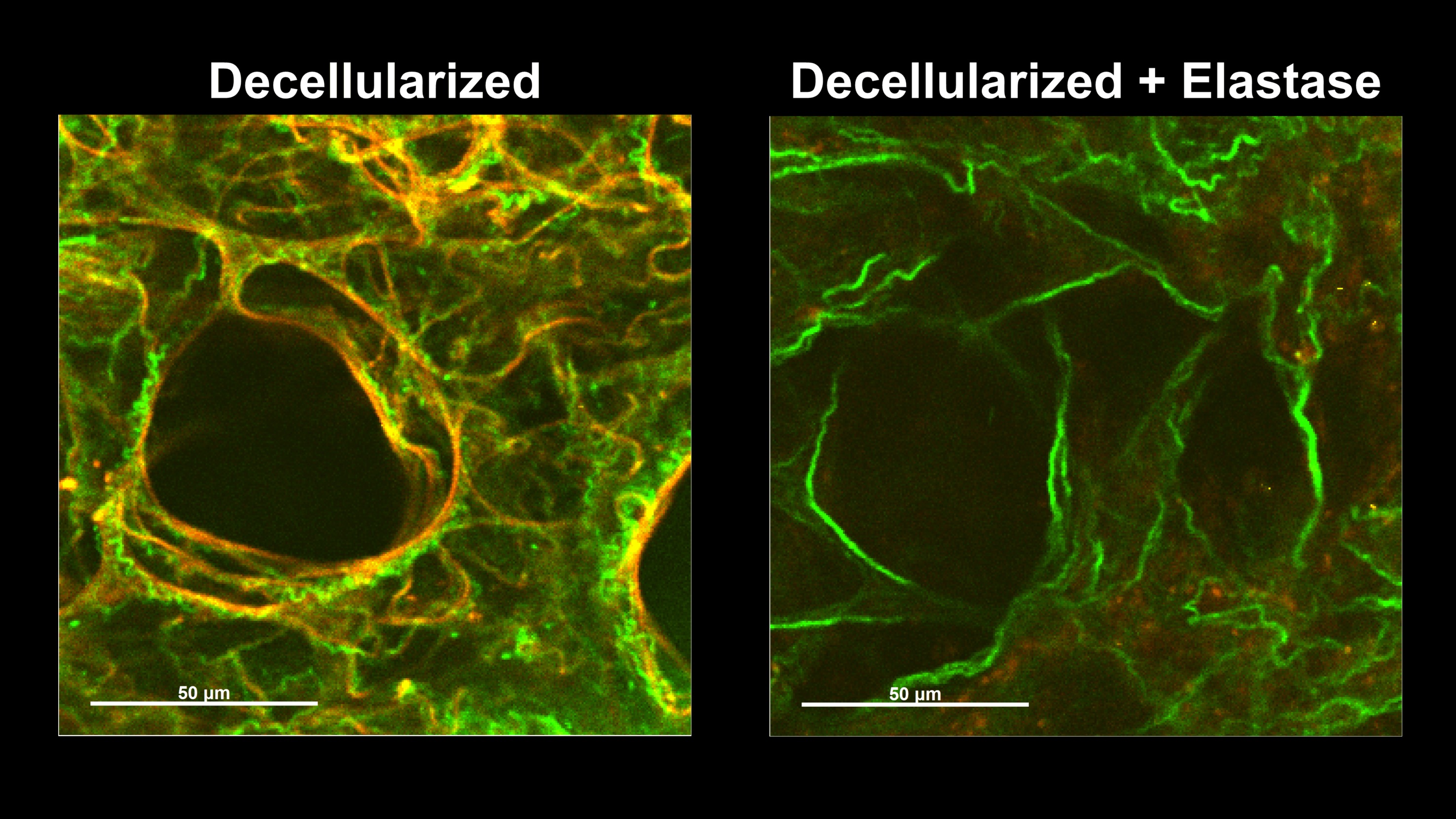
Collagen Without Elastin
Elastin fibers typically maintain collagen in a wavy state at physiologic lung volumes, thereby delegating collagen to a protective role as a safety net.
Enzymatically degrading the elastin fibers results in a loss of collagen waviness or tortuosity.
Collagen fibers without elastin support are vulnerable to elongation, plastic deformation, and failure during lung inflation.
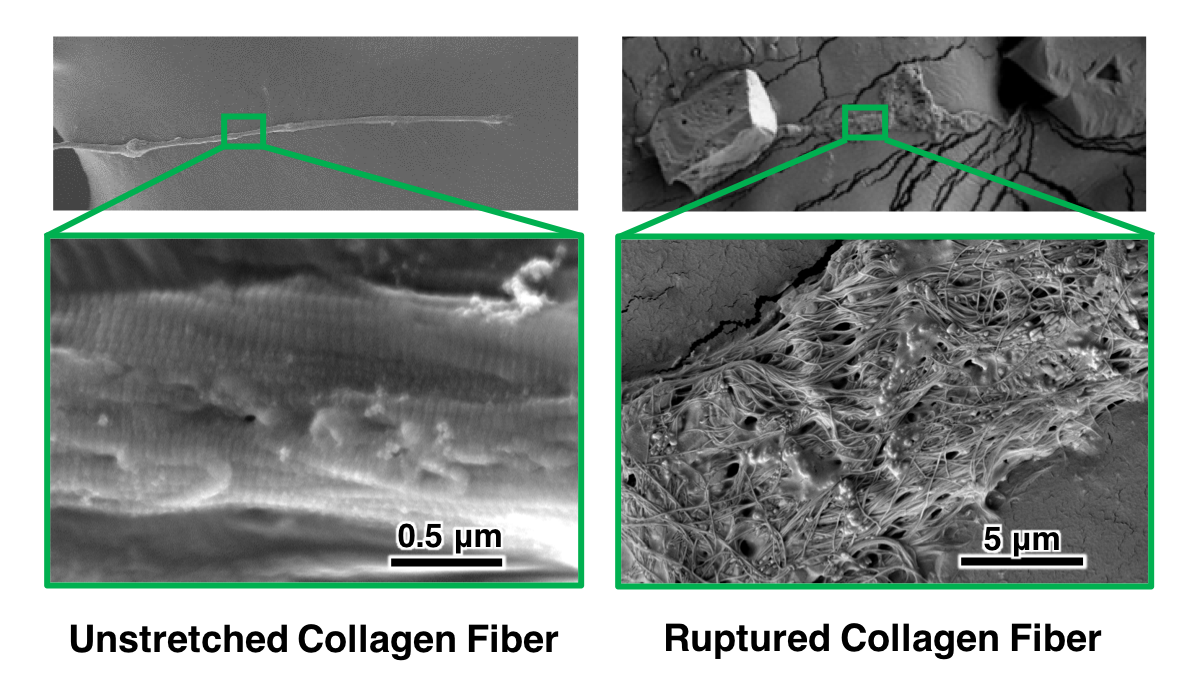
Collagen Fiber Nanostructure
Lung collagen fibers naturally exhibit a tightly packed architecture of smaller fibrils arranged in parallel.
After stretching to failure, collagen fibers exhibit plastic alterations: a loss of fibril organization and fibril-fibril crosslinking.